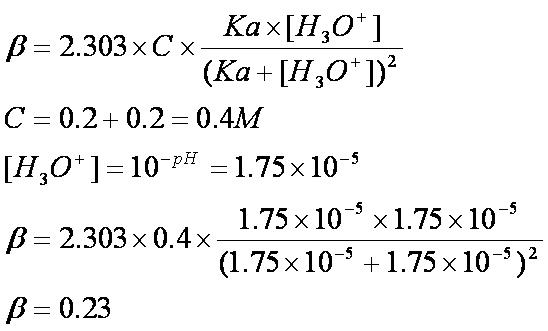Ph Of Buffer Solution Examples
Yes the cells of our body will not function.

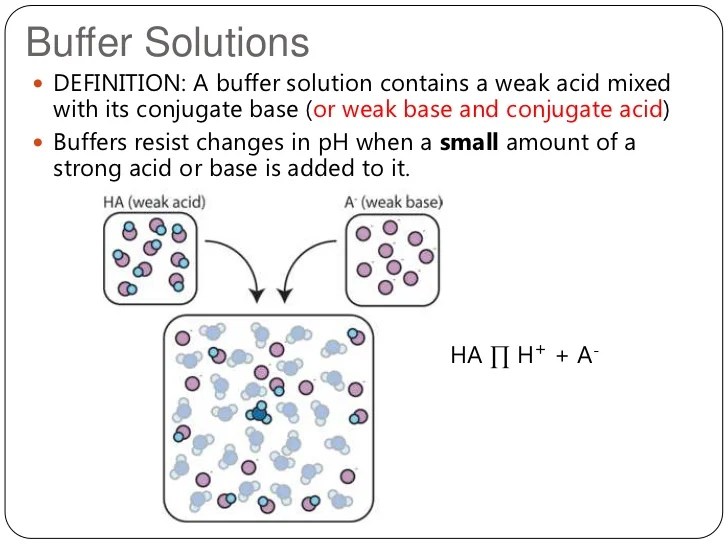
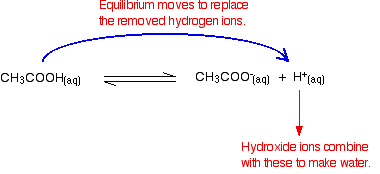
Ph of buffer solution examples. An acidic buffer solution is simply one which has a ph less. There are more examples. Suppose you wanted a buffer with a ph of 446. A buffer solution more precisely ph buffer or hydrogen ion buffer is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or vice versa.
What is a buffer solution. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Acidic buffer solutions. What are some examples of ph buffers.
Which creates a buffer solution. Some soaps also employ this strategy and so do some foodstuffs. You want only five why not 55. A buffer solution is composed of a weak acid and its conjugate base in appreciable concentrationsand so five examples are.
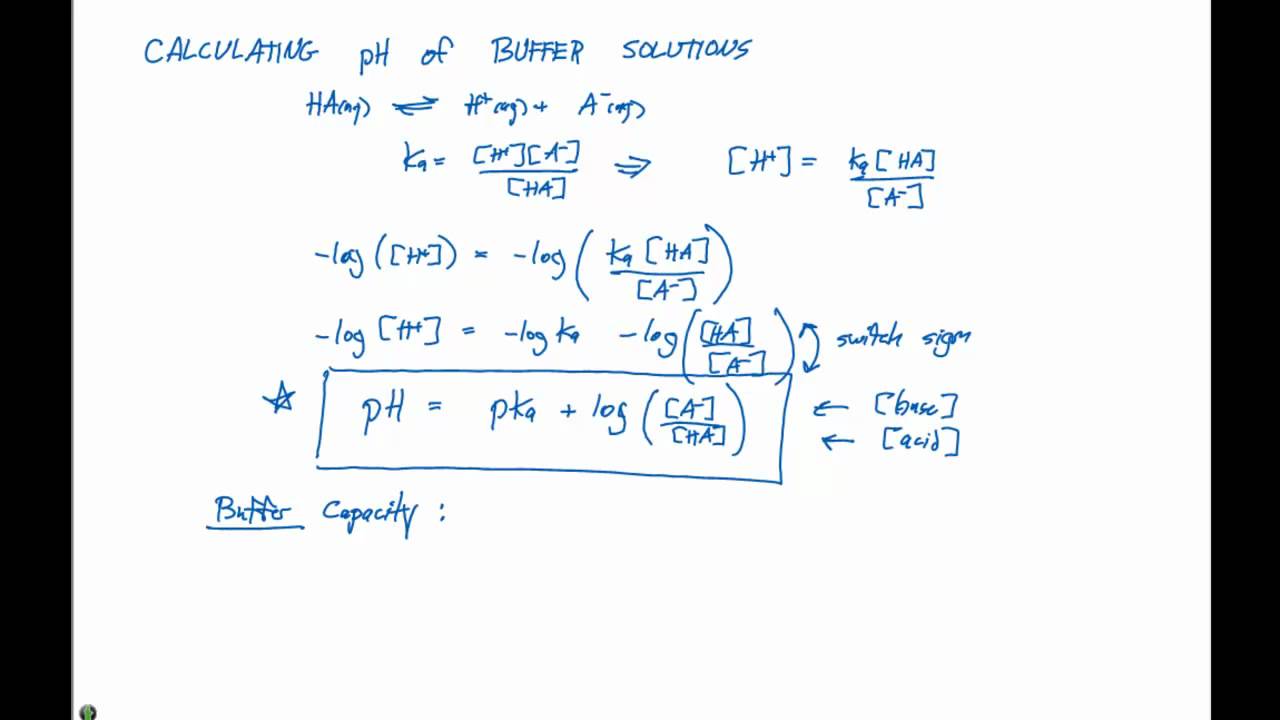
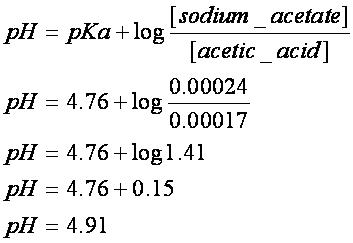
Example of calculating the ph of a buffer solution using the henderson hasselbalch equation including the ph of the buffer solution after adding some naoh. A good buffer solution will have roughly equal concentrations of both conjugate acid and conjugate base in which case its ph will be roughly equal to the pka or the. What do you think will happen if the ph of our blood changes drastically from its normal ph of 735.