Metal Oxide Plus Acid
Conversion of a metal oxide to the metal is called reduction.

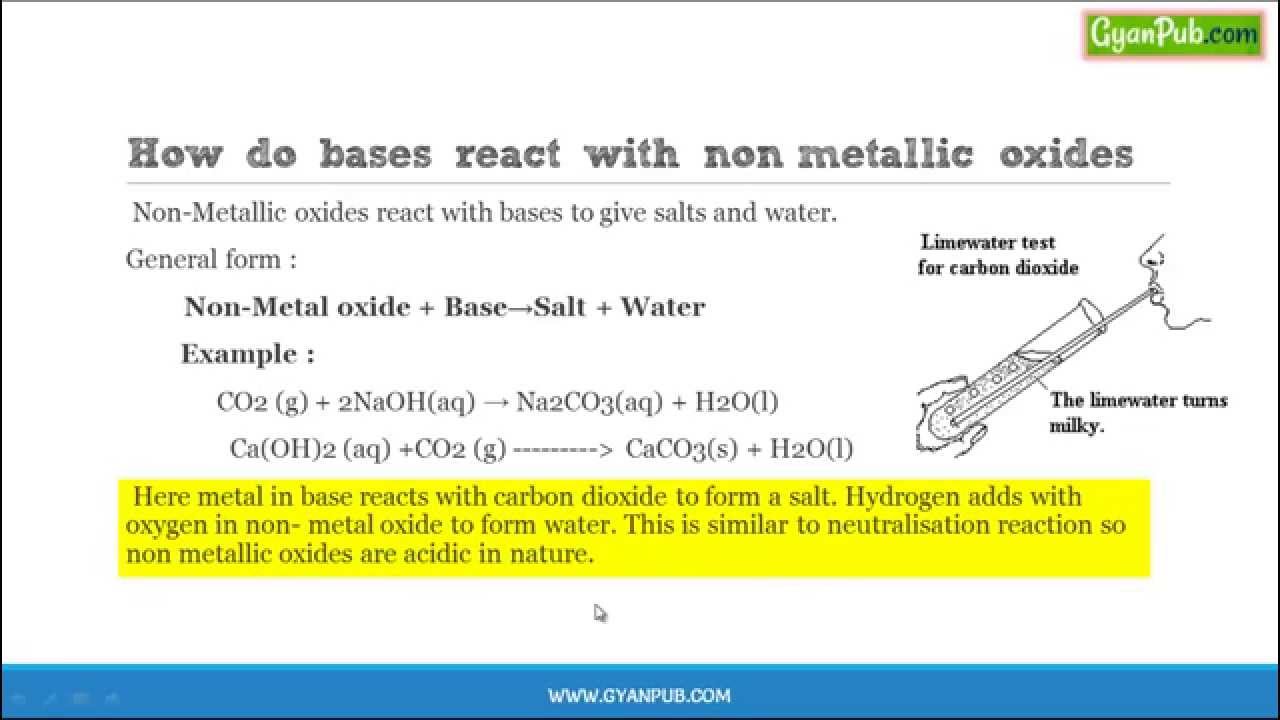
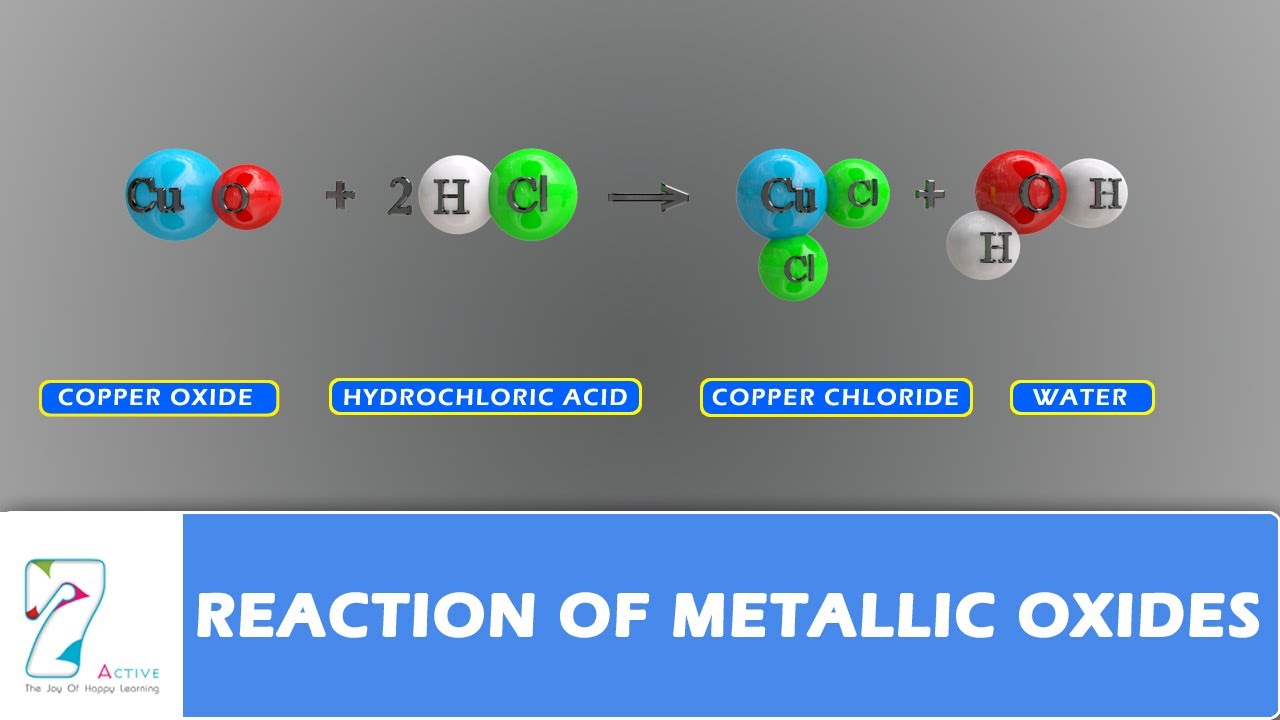
Metal oxide plus acid. How do acids reacts with metal oxides. The reaction between a metal oxide and an acid produces a salt and water. The resulting mixture is warm. The kind of salt.
Reaction of metal oxides with acid 1. Acids and metal oxides metal oxide acid 2. Acids and metal oxides metal oxide acid salt. In this experiment an insoluble metal oxide is reacted with a dilute acid to form a soluble salt.
Copperii oxide a black solid and colourless dilute sulfuric acid. A thermite reaction using ironiii oxide. A special case of the acid base reaction is the neutralization where an acid and a base. Some non metal oxides such as nitrous oxide n 2 o.
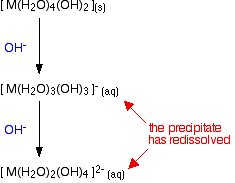
Is an amphoteric oxide. It can act as a base or acid. Silicon dioxide is a weakly acidic oxide. This metal oxide is feoh3 and is sparingly soluble in acetic acid.
Since this is the case could a reaction equation be generated. What does acetic acid do to feoh. Can u give an example of acid metal oxide salt water thanks.